A research article in the journal Drug Testing and Analysis uncovered a flaw in previous examinations of kratom’s main alkaloid, mitragynine.
The study, lead by University of Florida pharmacutics researcher Dr. Abisheak Sharma, stated:
There is a possibility that earlier reported studies did not separate the four mitragynine diastereomers, resulting in over‐quantification of mitragynine in these methods.
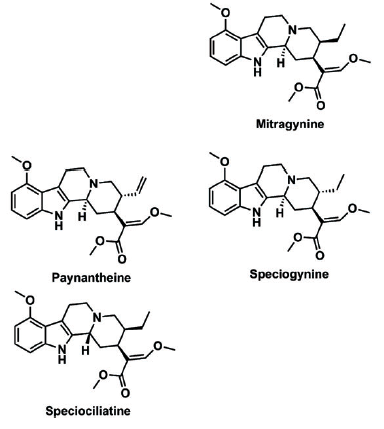
A diastereomer is a term in chemistry that describes a molecule that has a particular type of similarity in structure to other molecules. In this instance, other alkaloids with a similar molecular structure as mitragynine were misidentified as mitragynine.
the amount of mitragynine present in the samples analyzed by these methods might have overestimated the levels of mitragynine, including those utilized for determination of mitragynine content in human subjects…
Dr. Sharma told KratomScience, “If you look closely at mitragynine, speciogynine, speciociliatine, and paynantheine, by normal eyes you cannot find any difference. You need really good eyes to find a difference. These four molecules are diastereomers. So they are absolutely the same in their structure. They are like identical twins.”
Before this study, the previous method of liquid chromatography, a method in chemistry of separating elements so they can be observed, was not accurate enough to tell the difference between these four alkaloids.
When asked if this mistake may have led to false levels of mitragyinine in autopsy reports, Dr. Sharma told KratomScience, “Yes. There is a possibility. If I am taking a kratom product I should have all four diastereomers in my system. If I am not seeing four peaks in that chromatogram, it means they have merged all four to quantify it as mitragynine.”
In response to this oversight, researchers at University of Florida developed a more accurate chromatography, or in Dr. Sharma’s words, “really good eyes”.
The developed method reported herein, was able to separate various combinations of diastereoisomers (corynantheidine and isocorynantheidine; corynoxine and corynoxine B; mitragynine, speciociliatine, and speciogynine) with adequate resolution (≥ 2), which will be beneficial for future kratom research and analysis. The present method provides a significant advantage for the characterization of various kratom products for their alkaloid composition. This method is now regularly used for the analysis of kratom extracts and products intended for the pharmacokinetic and pharmacodynamics studies in our laboratory.
Falsely elevated levels of mitragynine in autopsy reports may have led medical examiners to overstate the role mitragynine played in deaths. Depending on the location, medical examiners may not have access to chromatography technology as accurate as the lab at University of Florida.
In the majority of the FDA’s reported “kratom-related” deaths, other substances, such as fentanyl, were found in the system of the deceased. However, another study co-authored by Dr. Sharma demonstrated that mitragynine may inhibit metabolism of some other drugs in the liver, leading to adverse drug interactions.
###